Thermodynamics
Thermodynamics, a science of the relationship between heat, work, temperature, and energy. In broad terms, thermodynamics deals with the transfer of energy from one place to another and from one form to another. The key concept is that heat is a form of energy corresponding to a definite amount of mechanical work.
Laws about Thermodynamics
A) Zeroth Law of Thermodynamics
Is Stated as "If two bodies P and Q are in thermal equilibrium and also P and R are in thermal equilibrium then Q and R are also in thermal equilibrium."
All bodies in thermal equilibrium are at equal temperature. Heat flows from the body of higher temperature to the body of lower temperature.
B) First Law of Thermodynamics
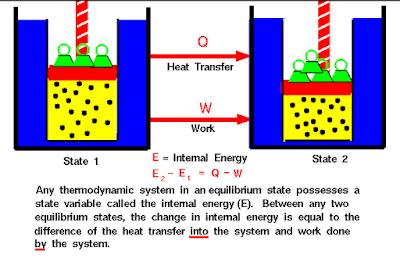
Heat and work are two distinct modes of energy transfer to a system that results in change in internal energy. Heat is energy transfer due to temperture difference between the system and the surrounds, whereas work is energy transfer brought about by means that do not involve any temperature difference
Let, ΔQ = Heat supplied to the system by the surrounding
ΔW = Work done by the system on the surrounding
Let, ΔQ = Heat supplied to the system by the surrounding
ΔW = Work done by the system on the surrounding
ΔU = Change in internal energy of the system
Definition-
"The energy (ΔQ) supplied to the system goes in partly to increase the internal energy of the system (ΔU) and the rest work on the environment(ΔW). "
Definition-
"The energy (ΔQ) supplied to the system goes in partly to increase the internal energy of the system (ΔU) and the rest work on the environment(ΔW). "
Formula
ΔQ = ΔU + ΔWC) Second Law of Thermodynamics
Mechanical work can be converted completely into heat but heat cannot be completely converted into mechanical work i.e. work and heat are not equivalent.
It can be stated in many ways
To design a heat engine working in a cyclic process is not possible and whose result is to take heat from a body at a single temperature and convert it completely into mechanical work.
A single temperature can be the temperature of the surrounding. All attempts to construct 100% efficient engine failed.
D) Third Law of Thermodynamics
The Third Law of Thermodynamics is concerned with the limiting behavior of systems as the temperature approaches absolute zero.
Definition "The entropy of a perfect crystal is zero when the temperature of the crystal is equal to absolute zero (0 K)."
At zero kelvin the system must be in a state with the minimum possible energy, thus this statement of the third law holds true if the perfect crystal has only one minimum energy state. Entropy is related to the number of possible microstates, and with only one microstate available at zero kelvin the entropy is exactly zero.
Adiabatic Process and Isothermal process
Definition - "An adiabatic process is any process occurring without gain or loss of heat or fixed temperature within a system."
Now assume these penguins to be atoms. Analogous to the penguins, at a temperature of zero Kelvin the atoms in a pure crystalline substance get aligned perfectly and do not move around. Matter is in a state of maximum order (least entropy) when the temperature approaches absolute zero (0 Kelvin). In other words, the entropy of a perfect crystal approaches zero when the temperature of the crystal approaches 0 Kelvin. This is, in fact, the third law of thermodynamics. The consequence of this law is that any thermal motion ceases in a perfect crystal at 0 K. Conversely, if there will be any thermal motion within the crystal at 0 K, it will lead to disorder in the crystal because atoms will start moving around, violating the third law of thermodynamics.





No comments:
Post a Comment